ABSTRACT
Objective: We present a case of a 22-year-old bodybuilder diagnosed with myocarditis secondary to clenbuterol use.
Results: The patient was primarily managed conservatively by the discontinuation of clenbuterol and the temporary use of dual anti-platelets, beta-blockers and nitrates.
Conclusion: Clenbuterol is a long-acting beta-2 agonist primarily used in veterinary medicine. In recent years, it has been illegally marketed as a weight loss supplement because of its anabolic properties and is popular among fitness enthusiasts. It is our aim to use this case to underscore the adverse effects of this drug with hopes that tighter regulations will be instituted to stem its illegal distribution.
LEARNING POINTS
- Clenbuterol is primarily a veterinary drug with bronchodilator and tocolytic properties.
- It is illegally used as a performance enhancer by athletes and bodybuilders because of its anabolic properties.
- Clenbuterol misuse can result in myocardial injury.
KEYWORDS
Clenbuterol, myocardial infarction
INTRODUCTION
Chest pain with concurrently elevated cardiac enzymes in a young patient with no known cardiovascular risk factors should prompt an exploration of the drug history. In recent years, anabolic steroids and weight loss supplements have started to gain a reputation for cardiac toxicity. Clenbuterol is 1 such drug, which has become popular amongst athletes and bodybuilders because of its anabolic properties. This drug is classified as a banned substance by sporting organizations and its use as a performance enhancer is illegal [1, 2]. However, it is not a regulated drug and is widely available for purchase online and in health food stores [1, 3]. This case report seeks to illustrate the adverse cardiovascular effects associated with clenbuterol. It is believed that as more cases emerge in the literature documenting complications associated with the misuse of this drug, policies will be developed towards the regulation of its use.
CASE DESCRIPTION
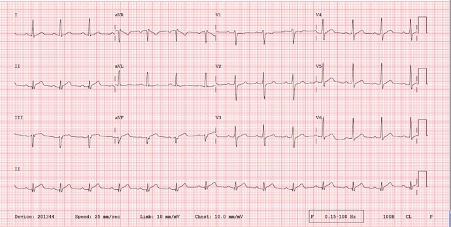
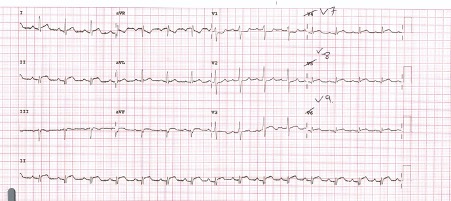
A 22-year-old male with no comorbidities presented with a 3-day history of intermittent, central chest pain. On the day of presentation, he had 2 episodes of chest pain at rest of a few hours’ duration and occurring a few hours apart. It was associated with 3 episodes of vomiting. There were no other significant symptoms. The patient was a bodybuilder who started taking clenbuterol 1 week prior. He took the lowest recommended dose of 40 micrograms but was unforthcoming about the dosing frequency. He denied taking other performance-enhancing or recreational drugs. He did not smoke and drank alcohol socially. He was haemodynamically stable, and his physical examination was unremarkable. Electrocardiogram (ECG) changes included ST depression with slightly upright T waves in V1 (Fig. 1). His baseline and 1-hour troponin I levels were both greater than 25,000 ng/l. The rest of the investigations were normal and SARS-CoV-2 was not detected. The patient’s chest pain had resolved by the time he presented to the hospital and an urgent echocardiogram did not demonstrate any structural or regional wall motion abnormalities and so he was managed conservatively with analgesics, antiemetics and intravenous fluids. Within 24 hours of admission, he had 2 further episodes of chest pain and vomiting. A computed tomography (CT) angiogram of the aorta ruled out aortic dissection. A repeat ECG confirmed ST changes in keeping with a posterior myocardial infarction (MI) with inferolateral involvement (Fig. 2) and an urgent inpatient coronary angiogram was arranged. Prior to the procedure, the patient was treated with intravenous metoprolol for a tachycardia of 120 beats per minute. A brief echocardiogram carried out immediately before cardiac catheterization demonstrated regional wall motion abnormalities affecting the lateral and posterior basal segments. However, the results of the angiogram demonstrated that the coronary arteries were normal in appearance.
Figure 1. ECG showing ST depression with slightly upright T waves in V1
Figure 2. Repeat ECG demonstrating an increase in R wave height, ST depression and upright T waves in V1-2. There were changes in keeping with posterior STEMI with inferolateral involvement
The patient was initially diagnosed with Type II MI secondary to clenbuterol-induced coronary vasospasm and commenced on bisoprolol, aspirin, ticagrelor and nitrates. Subsequently, outpatient cardiac magnetic resonance imaging (MRI) revealed delayed epicardial enhancement at basal to apical inferior segments and basal to mid lateral wall with myocardial oedema at the lateral and inferior wall of the left ventricle (LV) with normal biventricular systolic function in keeping with myocarditis. The previously prescribed medications were discontinued. He was briefly readmitted a few months later with a similar type chest pain while exercising but investigations did not support progressive myocardial injury and he was discharged with advice regarding exercise and strenuous activities and further outpatient follow-up was arranged. He remains well.
DISCUSSION
Clenbuterol is a long-acting beta-2 receptor agonist that is 100 times more potent than salbutamol [2]. Worldwide, it is primarily used as an equine bronchodilator and a bovine tocolytic, but it is still licensed for human use as a bronchodilator in certain countries [1]. It is well absorbed orally with a bioavailability of 70–80% and a long half-life of 25–39 hours [1,2 4]. Clenbuterol also has anabolic effects which makes it popular among bodybuilders [1]. Firstly, it promotes lipolysis through adipocyte beta-3 adrenoreceptors, and secondly, it leads to muscular hypertrophy by increasing protein synthesis in muscle fibres [1]. As a bronchodilator, the recommended oral dose is 20–40 micrograms twice daily [1,5]. There is no standardized dosing regimen for increasing lean muscle mass, and so, it is through trial and error that bodybuilders achieve the desired effects [1]. The most commonly documented dosing regimen among athletes is up to 200 micrograms, 1–3 times daily [1], which can be up to 5–10 times more than the dose required for asthma. Therefore, at these significantly higher doses, the risk of adverse effects increases. These include, but are not limited to, tachycardia, chest pain, vomiting, hypokalaemia, rhabdomyolysis, hypoglycaemia, agitation, hallucinations and convulsions [1,4,5].
Our patient had clinical, biochemical and radiological evidence of myocardial injury. In truth, the underlying pathophysiology by which clenbuterol exerts its toxic effects on the myocardium is not well understood. Several theories are in circulation in the literature. One author proposed that the use of clenbuterol can lead to ventricular hypertrophy with a subsequent increase in oxygen demand that culminates in myocardial injury [6]. Concerning our patient, there was no evidence of hypertrophic changes on cardiac imaging. However, the lack of ventricular hypertrophy may be explained by the short duration of clenbuterol use. In the animal study by Burniston et al. exploring the myotoxic effects of clenbuterol on the heart of a rat, myocardial necrosis was noted 4 hours after a single administration of clenbuterol subcutaneously. Myocardial necrosis was significantly reduced in the rats pre-treated with a beta-blocker, indicating that clenbuterol is directly toxic to the myocardium [7]. Furthermore, the study discovered that myocardial necrosis is not evenly distributed and tends to favour the LV, with maximal necrosis occurring 2.4 mm from the apex [7]. Shafrir et al reviewed nine case reports of clenbuterol-induced myocardial injury and the inferior and lateral ECG and ECHO changes were akin to the findings of the aforementioned study [4]. Interestingly, our patient’s ECHO and cardiac MRI regional findings also paralleled the distribution of myocardial necrosis in the study. Burniston et al. additionally proposed that clenbuterol’s myotoxic effects stems from its unfavourable effects on taurine levels, and this amino acid is cardioprotective as it maintains calcium homeostasis within myocytes [8].
Our patient did not have any other risk factors for myocardial injury, making clenbuterol the most likely culprit, although the mechanism by which it occurred is unresolved. Finally, there is no known antidote for clenbuterol poisoning and management is supportive [2]. Fluid resuscitation and vasopressors are recommended for hypotension and benzodiazepines for seizures and agitation. Hypoglycaemia should be corrected, and potassium replaced only if severe hypokalaemia is present, to avoid rebound hyperkalaemia, as the hypokalaemia is secondary to an intracellular shift [2]. Beta blockers may be administered for suspected myocardial ischaemia or infarction [2]. The ease of acquiring this drug and its popularity among fitness enthusiasts portends more incidents of clenbuterol toxicity. This is alarming given that some of its side effects may be associated with significant morbidity and mortality.
CONCLUSION
There is growing concern about the unrestricted use and availability of clenbuterol as it has a number of adverse effects when misused. Myocardial injury is just 1 of the potentially life-threatening complications associated with its use. Several theories have been postulated in the literature, but the underlying mechanism of myocardial injury remains unclear. There is no known drug that can counteract the effects of clenbuterol toxicity and management remains supportive.