ABSTRACT
Renal artery thrombosis is a threatening clinical diagnosis, in which renal infarction may occur. Often misdiagnosed, as it can mimic other common diseases, it should be considered in persistent flank pain to improve care and reduce morbidity. We review a case of a healthy 57-year-old woman with renal artery thrombosis mimicking pyelonephritis and renal calculus obstruction, highlighting the features of this clinical condition. An accurate diagnosis is essential for optimal management and prompt treatment, which remain to be defined.
LEARNING POINTS
- Although renal infarction is not rare, clinical diagnosis is often missed because of its non-specific presentation.
- Persistent abdominal/flank pain, elevated serum LDH and haematuria are the clues to the diagnosis of renal infarction.
- Early diagnosis and optimal thrombolytic treatment may sometimes restore renal function without the need for surgical intervention
KEYWORDS
Renal artery, thrombosis, flank pain
INTRODUCTION
Thrombosis of the renal artery is not as rare as previously assumed[1]. Series of cases report a higher incidence in autopsy (200 patients) when compared with clinical diagnosis (<50 patients), demonstrating misdiagnosed patients[1,2]. Therefore, in the setting of persistent and unexplained unilateral flank or abdominal pain, associated with haematuria, leucocytosis and especially an elevated LDH, diagnosis is strongly supportive[2-4]. Even when another more common renal disease is diagnosed, such as nephrolithiasis or pyelonephritis, the possibility of this condition should be considered.
Previous thromboembolic events, cardiac disease, chronic atrial fibrillation, trauma and aorta interventions are the most commonly associated risk factors. However, septic emboli from endocarditis, sickle cell disease, antiphospholipid syndrome, combined activated protein C resistance and protein S deficiency must also be investigated[2,4].
Renal ultrasound is usually the first exam to be made to exclude obstructive uropathy, however, if hydronephrosis is not present and this diagnosis is suspected, computed tomography (CT) angiography is essential to confirm the absence of perfusion[4]. Guidelines in re-establishing perfusion remain in discussion; standard anticoagulation with or without thrombolysis or surgical revascularization are the most frequent approaches. The extension of the embolism, time to diagnosis and the severity of renal failure may guide anticoagulation or cirurgical intervention.
CASE REPORT
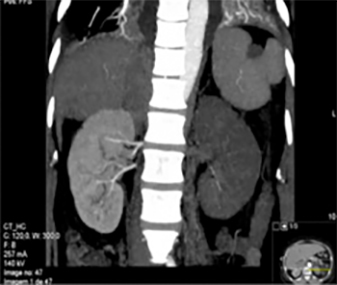
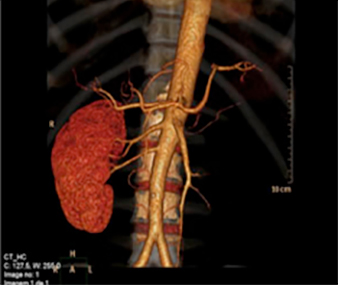
A 53-year-old woman with partial thyroidectomy subsequent to goitre was admitted to the emergency room with nausea, vomiting and persistent left lower abdominal pain for 3 days. Physical examination revealed a temperature of 37.8ºC, blood pressure of 148/78 mmHg, pulse 87/min, respiratory rate of 14/min and 98% oximetry. There was no abnormality in cardiac or vesicular murmurs. The abdomen was distended with tenderness over the left flank, with normal bowel sounds. Laboratory data showed leucocytosis (26,300 U) with 85% neutrophils and mild elevation of C-reactive protein (9.63 mg/dl), creatinine (1.10 mg/dl), aspartate aminotransferase (142 U/l) and alanine aminotransferase (109 U/l). Lactate dehydrogenase was high (1004 U/l). Urinalysis showed mild proteinuria and haematuria. Renal ultrasonography excluded obstructive uropathy and hydronephrosis. Under suspicion of a urinary tract infection, she started amoxicillin and clavulanic acid 1.2 g three times daily, for 24 h. Because of persisting abdominal pain, fever and high lactate dehydrogenase, an angio-CT scan of the abdomen was performed and revealed extensive renal thrombosis of the left renal artery (Fig. 1).
Fig. 1 - Angio-CT scan reveals left kidney infarction with thrombosis of the left renal artery (no perfusion seen in the left renal artery)
Considering the long delay in diagnosis, it was decided to carry out conservative treatment with heparin for 10 days, followed by oral anticoagulation with warfarin. Her clinical condition improved steadily, with fever and left flank pain regression. A doppler ultrasound of the kidney before discharge revealed maintenance of the left renal occlusion, with compensatory right kidney function. Despite extensive investigation, the cause of this thrombotic phenomenon was not established (Table 1).
| Haematology/biochemistry | Reference range, adults | 3rd hospital day |
|---|---|---|
| Erythrocytes | 3,630 | 3,900–5,000 U/l |
| Haemoglobin | 11.5 | 12.0–16.0 g/dl |
| Platelets | 234,000 | 150,000–400,000 U/l |
| White blood cells | 28,700 | 4,000–10,000 U/l |
| Neutrophils | 24,770 | 1,500–7,500 U/l |
| Urea | 29 | 21–43 mg/dl |
| Creatinine | 1.1 | 0.6–1.0 mg/dl |
| AST/ALT | 142/109 | 15-37/30-65 U/l |
| FA/GGT/Bilirubin | 56/34/0.1 | 50–136/5–55 U/l / <1.1 mg/dl |
| LDH | 1,004 | 81–234 U/l |
| Creatinine kinase | 405 | 26–192 U/l |
| C-reactive protein | 9,6 | <0.6 mg/dl |
| INR/aPTT | 1.0/23.6 | 0.8–1.2/24.3–35 seg |
| Electrophoresis | Normal | – |
| Antithrombin III | 25.0 | 20.0–40.0 mg/dl |
| C-protein antigen/activity | 68/82 | 72–150/70–140% |
| S-protein antigen/activity | 59/58 | 57–112/60–140% |
| Factor V/Factor V Leiden | 102/2.9 | 50–150%/>1.5 |
| Homocysteine | 17.3 | 5–18 umol/l |
| Plasminogen/Tissue plasminogen activator | 139 | 75–150% |
| Fibrinogen | 720 | 169–515 mg/dl |
| Antinuclear antibodies (includes anti-Ro antibodies, anti-La antibodies, anti-Sm antibodies, anti-nRNP antibodies, anti-Scl-70 antibodies, anti-dsDNA antibodies, anti-histone antibodies, antibodies to nuclear pore complexes, anti-centromere antibodies and anti-sp100 antibodies) |
Negative | – |
| Antiphospholipid antibodies (includes lupus anticoagulant and anticardiolipin antibody) | Negative | – |
| Anti-neutrophil cytoplasmic antibodies | Negative | – |
| Urine | ||
| Protein | ++ | – |
| Erythrocyte | +++ | – |
| Leukocyte | None | – |
Table 1 - Patient's laboratory tests
DISCUSSION
The diagnosis of renal artery occlusion is often delayed or missed due to its rarity and non-specific clinical presentation. The time to diagnosis following presentation is often more than 2 days, with fewer than 50% of cases being diagnosed promptly[5]. Symptoms vary depending on the type of disease and degree of involvement. Acute and constant abdominal pain and/or acute hypertension associated with high laboratory levels of white blood cells, serum creatinine and especially LDH and haematuria are the clues for diagnosis[1,2,4].
Serum LDH is a characteristic marker for cell necrosis, being high in about 99% of patients with renal infarction. It is not specific to this disorder, also being elevated in acute myocardial infarction, mesenteric embolism and haemolysis[2-4].
The mean age for presentation is 65 years, with no significant gender or kidney predominance. Bilateral involvement is seen in about 10% of cases[3,4].
Renal angiography is the gold standard exam, being performed later in the investigation. First, an ultrasound often rules out renal stones and, with higher sensitivity, a CT scan is performed when abdominal pain persists to exclude/suggest gastroenterological, gynaecological or other renal disorders that are more common[2].
Thromboemboli and in situ thrombosis are the two major causes for renal infarction. Other less common causes include trauma, vascular disorders, fibromuscular dysplasia, hypercoagulable states, aortic dissections and iatrogenic interventions. In our case, none of these had been established[3]. There is no consensus on first-line treatment since thrombolysis, anticoagulation and embolectomy may minimize the loss of renal function, and there are no prospective studies to compare them[5]. Patients typically start anticoagulation with intravenous heparin and oral warfarin, which help to prevent further embolic events. Perfusion can be reestablished through thrombolysis or surgical revascularization. The former is generally favoured as surgery has a higher mortality rate and does not result in better outcomes[5]. The reasons for the favourable outcome of conservative management are not completely clear, but the presence of collateral blood vessels and the activation of fibrinolytic systems may be contributing factors. The real benefit of continuing anticoagulation is unknown. However, data regarding repeated embolic/thrombotic events recommend anticoagulation indefinitely, especially in the setting of atrial fibrillation[5].
The endovascular treatment techniques include intra-arterial fibrinolytic therapy and mechanical thrombectomy with or without angioplasty. They are not standard with several fibrinolytic agents being applied. They are usually reserved for patients who do not have prolonged ischaemia time, cortical atrophy and contraindication for thrombolysis. There is no consensus regarding this issue, and the exact role of endovascular therapy remains to be defined[5].
Loss of kidney function and persistent hypertension are the most common sequel to renal infarction. Nevertheless, the majority of patients recover to normal with no permanent hypertension; only a small percent (8%) need dialysis[4].
Due long time to diagnosis the chosen approach was anticoagulation and no cirurgical intervention. She has recovered renal function, but the left kidney remains occluded. We highlight the need to consider this diagnosis to reduce delays in management and promote patients' care.