ABSTRACT
We report a case of a 55-year-old woman who was evaluated for multipleepisodes of late postprandial hypoglycaemia. We diagnosed her conditionas insulin autoimmune syndrome (Hirata disease) because of a highinsulin autoantibody (IAA) titre in association with high levels ofplasmatic insulin and hypoglycaemia in a patient with no history of exogenousinsulin administration and the exclusion of other causes of late postprandial hypoglycaemia.
LEARNING POINTS
- Hirata disease is a rare case of hypoglycaemia.
- Frequently, a metabolic syndrome due to chronic hyperinsulinism is associated.
- A correct diagnosis may spare a patient from an unnecessary surgical procedure.
- Therapeutic strategies are focused on decreasing glucose-dependent insulin secretion in the first phase of thepostprandialperiod.
KEYWORDS
Insulin autoimmune syndrome, hypoglicemia, hirata disease
INTRODUCTION
Insulin autoimmune syndrome (IAS) is defined by the presence of hypoglycaemia and autoantibodies against endogenous insulin in subjects without a history of exogenous insulin administration. This disorder was first described in Japan by Hirata et al. in 1970[1]. The prevalence of the syndrome is still unknown; the majority of the cases were described in Japan,where 244 cases were detected in the period 1970–1997[2]. To date, it represents the third leading cause of hypoglycaemic disorder in that country[2].This disorder is even more uncommon in non-Asian people, and few cases are described in the European and American literature[3]. In this paper, we report a case of Hirata disease in a middle-aged Italian woman.
CASE REPORT
A 55-year-old woman was referred to our clinic with metabolic syndrome. Routineblood examinations done 3 weeks before showed mild hyperinsulinaemia, impaired glucose tolerance and hypercholesterolaemia, and aglycosylated haemoglobin of45 mmol/l. She had a family history of diabetes mellitus. She had never smoked or drunk alcohol and had no allergies. She had ah istory of a thrombophilic syndrome (factor V Leiden heterozygote mutation) and postmenopausal osteoporosis. She had no history of diabetes,gastric surgery or pancreatic disorders. She had been taking aspirin andalendronate. She had never taken insulin or other oral hypoglycaemic agents.Her weight was 84 kg, body mass index 34.5 kg/m2and waist circumference 103 cm, consistent with a diagnosis of metabolicsyndrome. Otherwise, her physical examination was normal. The patient was discharged with nutritional and lifestyle change advice. In the following months, she began suffering from general fatigue, dizziness and palpitations that occurred about three hours after meals. A random measurement of capillary glycaemia during symptoms revealed aglucose level of 40 mg/dl. Laboratory evaluation revealed normal blood cell counts,blood chemistry and fastingglucose. She also had normal levels ofadrenocorticotropic hormone (ACTH), cortisol, thyroid-stimulating hormone (TSH) and glycosylatedhaemoglobin, but abnormally high levels of fasting insulin and c-peptide (Table 1). No abnormalities were detected in any other autoimmunity markers (Table 1). The association of hyperinsulinism, weight gain and hypoglycaemia suggested the presence of an insulinoma.
| Time | Normal range | |
|---|---|---|
| White blood cells | 6980/mmc | 4,000–11,000/mmc |
| Haemoglobin | 13.4 g/dl | 12–16 g/dl |
| Platelets | 260,000/mmc | 150,000-400,000 mmc |
| Fasting insulin | 72μUI/ml | 3–25 μUI/ml |
| Fasting C-peptide | 2.53 ng/ml | 0.81–3.85 ng/ml |
| 8:00 a.m. cortisol | 549 nmol/l | 150–650 nmol/l |
| ACTH | 40 pg/ml | 5–49 pg/ml |
| HbA1c (HPLC) | 45 mmol/mol | <48 mmol/mol |
| Polymerization chain reaction | 3.32 mg/l | 0–5 mg/l |
| Antinuclear antibodies | Absent | - |
| Anti-dsDNA | Absent | - |
| Extractable nuclear antigen | Absent | - |
| Anti-SCL-70 | Absent | - |
| Anti-surreal-gland | Absent | - |
| Anti-GAD | Absent | - |
| Anti-iiInsulin receptor | Absent | - |
| Anti-insulin | >100 UI/l | - |
Table 1 - Laboratory findings in our patient
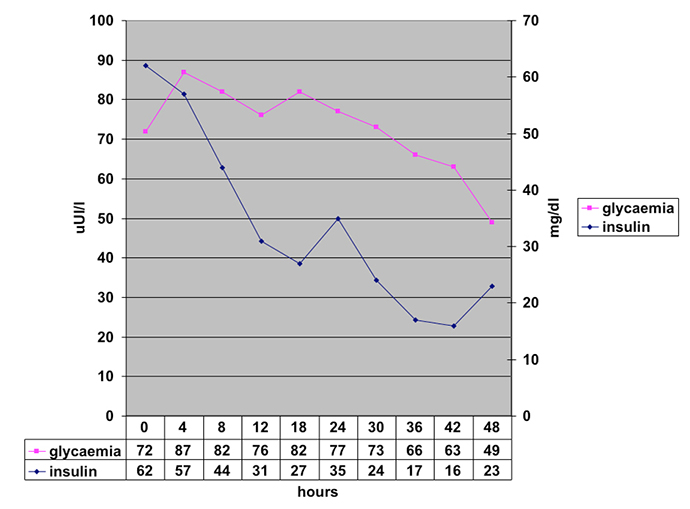
During 48 h of fasting, no hypoglycaemia occurred. However, serumconcentration ofinsulin and c-peptide progressively normalized (Fig. 1).
Fig. 1 - Blood glucose and insulin levels during prolonged (48 h) fasting test
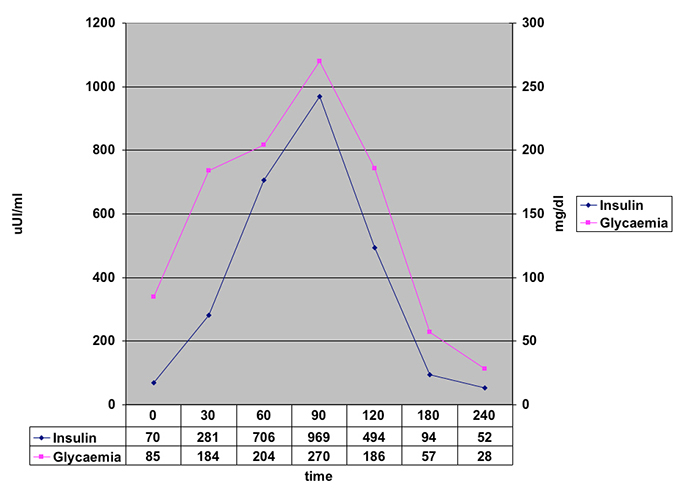
Subsequently, we performed an oral glucose tolerance test, which showed avery high concentrationof insulin in the late postprandial period in association with abnormally lower blood glucose levels (Fig. 2).
Fig. 2 - Blood glucose and insulin levels during oral glucose tolerance test
Since no pancreatic masses or other suspicious lesions were detected by ultrasound of the abdomen, or by thoracic and abdominal CT scans, we measured anti-insulin antibodies (IAA), which were present in high titres(IAA>100 UI/ml). The presence of a high insulin autoantibody titre in association with high levels of plasmatic insulin and hypoglycaemia without a history of insulin administration confirmed the diagnosis of autoimmune hypoglycaemic syndrome (Hirata disease).
DISCUSSION
IAS or Hirata disease is very uncommon in Western countries: to dateonly 60 caseshave been reported in the non-Asian population. It is clinically characterized by hyperinsulinaemic hypoglycaemic episodes, often occurring in the late postprandial period. Fasting- and exercise-induced hypoglycaemia has also been described. IAS has been associated with many comorbidities and exposure to various medications. The diseases most frequently associated with IAS are rheumatologic diseases such as systemic lupus erythematosus (SLE) and systemic sclerosis, and haematological diseases in which there is an improved production of immunoglobulins such as benign monoclonal gammopathy or multiple myeloma[3,4]. Positive antinuclear antibodies, anti-double-stranded DNA and rheumatoid factors in the absence of clinical manifestations are also common findings. Many drugs have been associated with the development of Hirata disease. Lupsa et al.suggested that in about 47% of the non-Asian patients, IAS seemed to be triggered by exposure to different medications (captopril, penicillamine, pyritinol, imipenem, hydralazine, procainamide, isoniazide, penicillin G)[3]. In the Japanese cases, a clear relation was demonstrated between medications containing the sulphydryl group and the occurrence of the syndrome3. Our patient did not show any rheumatologic or haematological disorder and had no history of exposure to the drugs mentioned previously.
PATHOPHYSIOLOGIC CONSIDERATIONS
Hirata disease is a rare cause of postprandial hypoglycaemiawhose mechanism is to date not completely understood. Some authors have suggested a strong association between IAS and human leukocyte antigen (HLA) haplotype. Most HLA-DR4-positive patients with IAS have HLA-DRB1*0406, DRB1*0403 or DRB10407. Moreover, it has beensuggested that agents such as sulphydrylcompounds (methimazole, carbimazole, penicillamine) may be related to the onset of the syndrome. It has been postulated that the sulphydryl group interacts with the disulphide bondsin the insulin molecule, making the insulin more immunogenic, either by hapten formation, or by cleavage of the disulphide bondsof the insulin molecule. In most cases, discontinuing the sulphydryl medications was associated with the remission of the hypoglycaemic episodes3. It has been hypothesized that hypoglycaemia occurs through two main mechanisms:
- During the early postprandial period, the autoantibody immediately binds to insulin,masking its bioactivity to its receptors in the liver and in peripheral tissues. This results in hyperglycaemia andfurther insulin secretion.
- During the late postprandial period, the autoantibody, because of low affinity,dissociates from insulin and the free insulinexerts its intrinsic glucose-lowering effect even in the presence of normal levels of glucose, thus causing hypoglycaemia.
The lower affinity and high binding capacity of IAA weredescribed by Eguchi[5] in 1998. Thus, the autoantibody easily binds to insulin, but the insulin–autoantibody complex becomes unstable and suddenlyuncouples once plasma insulin increases above certain threshold levels. In our case, this mechanism appears to have been present. In fact, in the OGTT test we observed that the insulin levels rose rapidly in the early postprandial period, independently of the blood glucose levels, and the glucose levels fell only in the late postprandial period (Fig. 1). We also observed that there was a degree of fasting hyperinsulinism that disappeared only after 48 h of fasting (Fig. 2).
THERAPEUTIC APPROACH
The most widely suggested therapeutic strategies for Hirata disease areeducational measures like frequent and small meals, low in simple carbohydrates. The use of alpha-glucosidase inhibitors has also been suggested. Both strategies are intended to decrease glucose-dependent insulin secretion in the first phase of the postprandial period, the former by reducing the glycaemic load and the latter by delaying glucose absorption. Administration of corticosteroids or immunosuppressive drugs in combination with plasmapheresis is another possible therapy, but clinical evidence islacking for these treatments. In our patient, acarbose was administered without improvementof postprandial glycaemia. Our choice, therefore, was to educate thepatient to eat low-carbohydrate meals, and this was enough to avoid her postprandial symptoms. In conclusion, Hirata disease is a very uncommon cause of hypoglycaemia, however it should be considered in the differential diagnosis in any patient with hyperinsulinaemic hypoglycaemia because a correct diagnosis may spare a hypoglycaemic patient from an unnecessary pancreatic surgical procedure.