ABSTRACT
Infectious purpura fulminans is a rapidly progressive skin necrosis that has a mortality rate of 30%[1,2]. Here, we describe a case of infectious purpura fulminans caused by Capnocytophaga, diagnosed by a blood film.
LEARNING POINTS
- C. canimorsus -induced septicaemia, though rare, should be considered in any patients presenting with sepsis.
- A history on animal exposure, previous foreign travel and recent contacts isvital.
- Waiting for blood culture could delay treatment. Alternative methods of diagnosisshould be considered.
KEYWORDS
Capnocytophaga, purpura fulminans, bacillus, sepsis
CASE REPORT
A previously healthy 66-year-old man presented to the emergency department with a history of lessthan 24 hours of feeling unwell, fever, non-productive cough and vomiting. He had not been exposed to anyone sick in the recent past and had no past medicalhistory.
On admission, he was pyrexic at 38.3°C, tachycardic at 125 beats per minute and tachypnoeic at 33breaths per minute. His blood pressure was 88/61 mmHg. On auscultation, there were minimal crepitations at both lung bases. All other physical examinationswere normal. Biochemistry results demonstrated a raised white cell count with left shift, acute kidney injury, deranged liver function and disseminatedintravascular coagulation (Table 1).
| Laboratory value | Reference range | |
|---|---|---|
| Biochemistry | ||
| C-reactive protein | 284 mg/l | <5 mg/l |
| Sodium | 143 mmol/l | 135–145 mmol/l |
| Potassium | 3.4 mmol/l | 3.5–5.0 mmol/l |
| Creatinine | 240 μmol/l | 45–120 μmol/l |
| eGFR corrected for ethnic group | 21 | >90 ml/min/1.73 m2 |
| Urea | 8.1 mmol/l | 3.3–6.7 mmol/l |
| Phosphate | 1.76 mmol/l | 0.80–1.40 mmol/l |
| Corrected calcium | 2.09 mmol/l | 2.15–2.60 mmol/l |
| Total protein | 66 g/l | 60–80 g/l |
| Albumin | 36 g/l | 35–50 g/l |
| Globulin | 30 g/l | 25-35 g/l |
| Bilirubin (total) | 26 μmol/l | 3–20 μmol/l |
| Alkaline phospatase | 159 IU/l | 30–130 IU/l |
| Aspartate transaminase | 515 IU/l | 10–50 IU/l |
| Gamma-glutamyl transferase | 251 IU/l | 1–55 IU/l |
| Haematology | ||
| White blood cells | 16 | 4–11×109 cells/l |
| Red blood cells | 4.08 | 4.5–5.8×1012 cells/l |
| Haemoglobin | 10.8 | 13–16.5 g/dl |
| Mean corpuscular volume | 88.2 | 77–95 fl |
| Platelets | 24 | 150–450×109cells/l |
| Neutrophils | 10.46 | 2.2–6.3×109 cells/l |
| Lymphocytes | 1.08 | 1.3–4×109 cells/l |
| Monocytes | 0.55 | 0.2–1.0×109 cells/l |
| Basophils | 0.06 | 0–0.1×109 cells/l |
| Fibrinogen | 5.5 | 1.5–4 g/l |
| INR | 2 | 1.0–1.5 |
Table 1 - Initial blood test results of the patient during admission
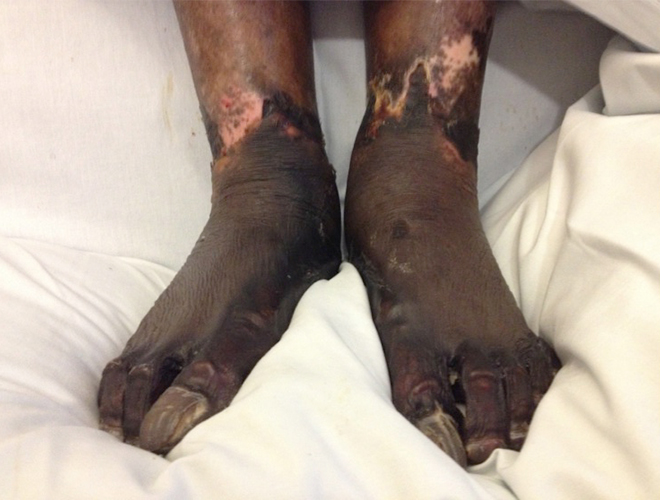
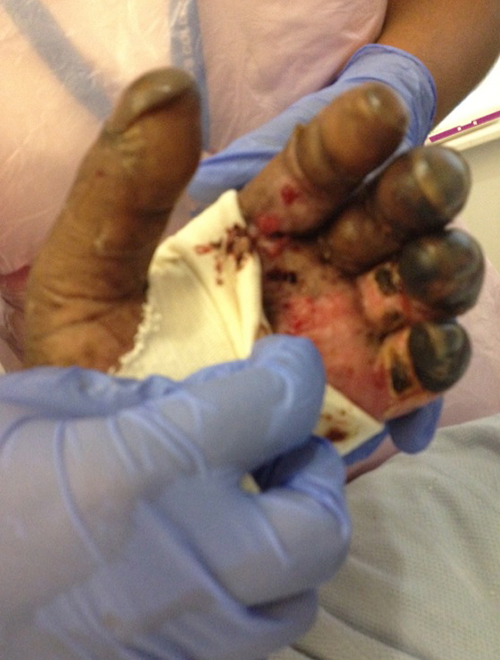
Chest radiograph showed possible right basal consolidation. A computed tomography ofhis chest, abdomen and pelvis showed features of an infarcted spleen, with no evidence of intra-abdominal collections. A diagnosis of sepsis of unknownorigin was made and intravenous Tazocin (piperacillin and tazobactam) was commenced at 4.5 g twice a day. Five litres of intravenous saline, 1 l ofcolloid and 15 ml/kg of fresh frozen plasma were administered. Continuous haemodialysis was instigated in the Medical Critical Care Unit (MCCU). Despiteall therapeutic efforts, the patient became progressively hypotensive and was intubated, mechanically ventilated and treated with milrinone andnoradrenaline. Within the first few hours in the MCCU, he developed cutaneous blistering, initially on the left arm, later progressing rapidly, forming areasof widespread ecchymosis involving the thorax, abdomen, digits, lower limbs and nose tip (Figs. 1 and 2).
Sepsisdue to meningococcal disease or staphylococcal/streptococcal infection was suspected and antibiotic treatment was escalated to intravenous clindamycin,ciprofloxacin, Tazocin and linezolid.
INVESTIGATION
The initial blood cultures showed no growth. These blood cultures were sent for prolongedincubation on chocolate agar and again no growth was detected. The urine culture and wound swab showed no growth. His retroviral test was negative. Thecombined nose and throat swab showed absence of influenza A, influenza B, respiratory syncytial virus, parainfluenza virus type 1, 2 and 3 andadenovirus. Cytomegalovirus, Epstein–Barr virus and hepatitis screens were negative. Sputum culture showed no acid-fast bacilli. Urine pneumococcalantigen was negative.
A second set of blood cultures grew Candida glabrata. The positive fungal culture suggested a possible occult infection although fundoscopy examination was normal and neither the transthoracicechocardiogram nor transoesophageal echocardiogram showed any vegetations.
The central venous line, peripheral line and nasogastric line tips were negativefor bacterial and fungal growth. Intravenous amphotericin B was added to the antibiotic regimen but it was agreed that the fungal growth was not the primaryfocus of infection.
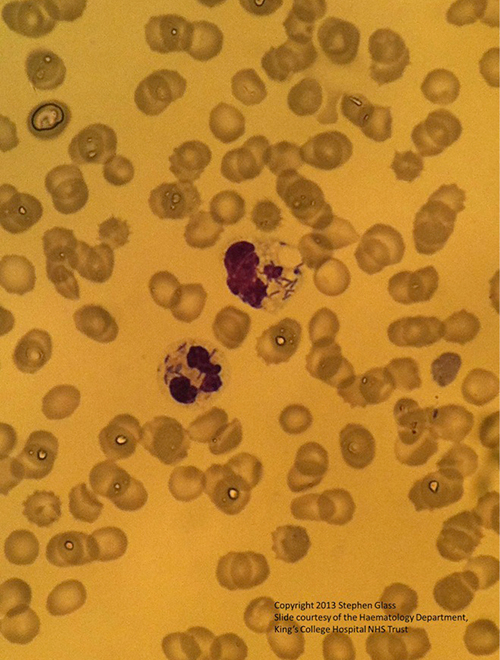
The focus of infection remained unclear, until results of a peripheral bloodsmear (Fig. 3) showed rod-like inclusions consistent with an intracellular bacterium, raising a suspicion of Capnocytophaga canimorsus septicaemia. This was supported by the patient’s history. A family dog hadbitten the patient 48 hours prior to the onset of symptoms; the bite marks were superficial. The dog was up to date with vaccinations. Antibiotic therapy waschanged to meropenam, given reports of beta-lactam-resistant strains of the Capnocytophaga species. The areas of ecchymoses on the nose, lower limbs and fingertips becamenecrotic, requiring surgical debridement.
Fig. 3 - A peripheral blood smear showing intracellular bacilli in neutrophils
OUTCOME
The patient had 14 days of intravenous meropenam. He eventually had bilateralbelow-knee amputations and became wheelchair bound, requiring ongoing rehabilitation.
DISCUSSION
C. canimorsus is a gram-negative, non-spore-forming bacillus found in the gingival flora of catsand dogs. It is transmitted to man by bites (54% of cases), scratches (8.5%) or by mere exposure to animals (27%)[2,3]. Human infection with this bacterium israre. It tends to occur at greater frequency in those who are immunocompromised (5%) and those with asplenia (33%), alcoholism (24%), chronic lung disease andcirrhosis[4,5]. Despite its low virulence, 50% of patients suffering from C. canimorsus septicaemia develop severe purpura fulminans. The mortality rate is 30% and prompt diagnosis is essential[3-5].
Patients with C. canimorsus infections present with nonspecific signs such as nausea, vomiting and shortness of breath and progress rapidly to septicshock. Few develop a maculopapular rash at the site of the animal bite[4,5]. Some patients develop haemorrhagic adrenal insufficiency[6]. There are also rare instances of C. canimorsus endocarditis or meningitis[7,8].
As C. canimorsus is a fastidious, slow-growing organism, isolation of this organism is difficult. Culturing of C. canimorsus involves using an enriched agar such as chocolate, 5% sheep blood, heart or brain–heart infusionagar with 5% rabbit blood at 37°C. Colonies may not be visible for up to 7 days. Even with meticulous culture conditions, blood cultures are negative in30% of cases[9,10]. Some authors have suggested identification of C. canimorsus through polymerase chain reaction (PCR) and 16s ribosomal RNA gene sequencing[11], but such facilities are not readily available in allcentres.
C. canimorsus infection can cause organ failure. The mechanisms involve widespread inflammatory response secondary to endotoxin production. Thesystemic influx of the local inflammatory mediators causes tissue toxicity, microvascular ischaemia and cell death[12]. Treatment involves early targetedantibiotic therapy and intensive organ support[12,13]. C. canimorsus infection responds well to penicillin and β-lactam–β-lactamase inhibitor combinations.Other active agents include clindamycin, linezolid, tetracycline, carbapenems and chloramphenicol[14]. Due to the increasing frequency ofβ-lactamase-resistant strains, meropenam was chosen[14,15]. Though monoclonal anti-endotoxin antibodies have been used in gram-negative sepsis, there arestill insufficient clinical studies to prove its benefit[17].