ABSTRACT
Arnold–Chiari malformation is defined as downward displacement of the brainstem and cerebellum through the foramen magnum. It has different clinical presentations and four subtypes. It is known that downward migration of posterior fossa components through the foramen magnum and associated lower cranial nerve palsy and brainstem compression can cause respiratory failure. Acute respiratory failure could mark the onset of thedisease. Posterior fossa decompression performed to treat primary disease can improve the central sleep abnormalities. As respiratory failure is rarely seen, this paper presents two cases of Arnold–Chiari malformation with respiratory failure.
LEARNING POINTS
- Arnold–Chiari malformation can cause central nervous system sleep abnormalities andrespiratory failure.
- In young patients with hypercapnic respiratory failure, neurologic causes must besought.
- In Arnold–Chiari malformation, respiratory problems may diminish or persistpostoperatively.
KEYWORDS
Arnold–Chiari malformation, respiratory failure, hypercapnia
INTRODUCTION
Arnold–Chiari malformation (ACM) is defined as downward displacement of the brainstem andcerebellum through the foramen magnum[1]. Patients are mostly referred to a physician with weakness, loss of sensation, bulbar dysfunction, headache,imbalance, gait disturbance and syncope, but also with acute respiratory failure[2]. Adolescents and adults who have ACM but no symptoms initially may,later in life, develop signs of the disorder. Respiratory failure is rarely seen, but it can be present at onset. Respiratory failure is mostly due tocentral hypoventilation and apnoea, although an obstructive pattern is occasionally reported[3,4]. It is known that downward migration of posteriorfossa components through the foramen magnum and associated lower cranial nerve palsy and brainstem compression can cause respiratory failure[3]. This paperreports two ACM patients with respiratory failure and sleep apnoea syndrome treated surgically.
CASE 1
A 35-year-old man, affected by ACM and syringomyelia, who had been operated on 4 years before, had onset of dyspnoea. Subsequently,diaphragm plication was performed for left diaphragm paralysis. The patient had been on Bilevel Positive Airway Pressure (BIPAP) treatment with pressure of11/5 mmHg at night for his hypercapnic respiratory failure. The patient was referred to our clinic with dyspnoea, long-lasting purulent sputum and ahistory of repeated respiratory infections. The physical examination revealed slow motion and slow speech. He was otherwise normal. His arterial blood gasanalysis in room air showed PaO2 80 mmHg, PaCO2 52 mmHg, pH 7.40 and SaO2 95%. A chest X-ray showed left diaphragmatic elevation.
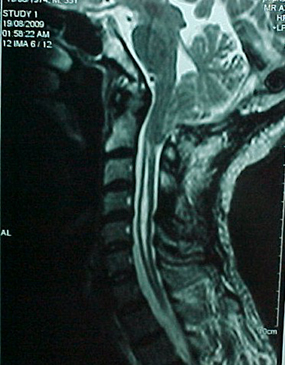
His lab values were normal. Forced Vital Capacity (FVC) was 2350 ml(50%), Forced Expiratory Volume 1 second (FEV1) 2280 ml (60%) and FEV1/FVC 0.97. The 6-minute walking test did not cause desaturation for a walkingdistance of 320 m. There was no additional pathology in cranial and whole spinal MRI (Fig. 1).
Polysomnography disclosed hypopnoea and apnoea periods of central origin that persisted withBIPAP 11/5 mmHg. The apnoea–hypopnoea index (AHI) was 31.4 (severe sleep apnoea syndrome), well controlled with 16/6 mmHg pressure.
Fig. 1 - Cranial MRI of the first case showing downward displacement of cerebellar tonsils and syringomyelia.
CASE 2
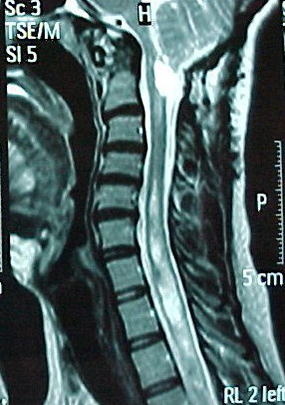
A 35-year-old female presented with sleepiness; swelling of legs, face and neck; cyanosis of fingers and lips; andright pleuritic chest pain. A physical examination showed bilateral +/+ pretibial oedema, jugular venous distention and decreased breath soundsbilaterally in the middle–lower pulmonary field. She had an abnormal gait with broad steps. Her past medical history disclosed posterior fossa decompressionsurgery for ACM and syringomyelia 9 years before. She was followed for a nonfunctional hypophysial adenoma. Her vital signs were normal. PaO2 was 31 mmHg, PaCO2 75 mmHg, pH 7.32 and SO2 61%. The patient was treated with BIPAP and oxygen for hypercapnic respiratory failurecausing hypoxia. The right costophrenic angle was blunted. The thorax CT demonstrated pneumonic infiltration in her middle pulmonary lobe, right pleuraleffusion and associated compression atelectasis. The lab exams showed C-reactive protein 15 mg/dl and no leukocytosis. Moxifloxacin 400 mg i.v.q.d.was started. FVC was 1970 ml (75%), FEV1 1340 ml (59%) and FEV1/FVC 0.68. During the 6-min walking test, there was no desaturation for a walking distanceof 280 m. Pulmonary arterial pressure (PAP) ranged between 35 and 45 mmHg and echo examination showed a dilated right atrium. Daytime SaO2 rose to 98% during Noninvasive mechanical ventilation (NIMV). With BIPAP 14/5 mmHg, PaO2 was 62 mmHg, PaCO2 74 mmHg, pH 7.45 and SO2 92%. The patient was allowed to breathe room air in the daytime, aided with BIPAP 14/5mmHg at night. In her cranial and whole spinal MRI, there was a cystic formation near the brain stem (Fig. 2), which could have caused brain stem compression and central hypoventilation,although brainstem auditory evoked potential (BAEP) was normal. Polysomnography demonstrated severe sleep apnoea (AHI 49.4) and desaturation, which improved withBIPAP 15/8 mmHg plus oxygen 2 l/min.
Fig. 2 - Cranial MRI of the second case showing a cystic formation near the brainstem and syringomyelia.
DISCUSSION
ACM is the elongation of the brain stem and cerebellum through the foramen magnum[1], which can damage central respiratory control, leading to respiratory disorders, respiratory failure and death[3]. The associated cervical syringomyelia can also lead to respiratory failure due tomuscular weakness and phrenic nerve disease. Posterior fossa decompression performed to treat the primary disease can improve central sleep abnormalities[3].Levitt and Cohn reported improvement in sleep apnoea after surgical treatment of ACM, but the AHI (14) was less severe than that of our patients[5].Improvement in both central and obstructive sleep disorders occurs postoperatively, indicating that ACM is responsible for both[4]. However,respiratory failure may not improve as much as sleep disturbance, such that patients may need NIMV and oxygen support. Both of the cases herein describedhad ongoing respiratory failure after surgery. BIPAP was introduced postoperatively, but higher-pressure NIMV was needed afterwards. The secondcase had respiratory failure triggered by pulmonary infection, as already reported by Alvarez et al.[3]
Sleep abnormalities of ACM can be adequately treated with NIMV (CPAP, BIPAP) at night-time, with oxygen supplementation, assuggested by polysomnographic studies. As the symptoms of this disease can be nonspecific, it is important to keep this in mind when dealing with central nervous system symptoms in young adults with hypercapnic respiratory failure. As in our cases, patients operated on for ACM must be evaluated for chronic respiratory failure and sleep apnoeaafter surgery, as respiratory problems can progress in time, avoiding respiratory arrest. As demonstrated by one of our patients, life-threateningrespiratory failure may occur after an infectious trigger in the absence of previous respiratory symptoms.