ABSTRACT
Objectives: To report a case of hypereosinophilic syndrome which presented clinically as acute coronary syndrome.
Materials and methods: We describe the case of a 69-year-old woman with acute coronary syndrome and peripheral hypereosinophilia.
Results: The condition rapidly evolved to severe heart failure. Coronary disease was excluded by cardiac catheterization. Systemic corticosteroid therapy was initiated and further secondary causes of hypereosinophilia were excluded.
LEARNING POINTS
- Since sudden worsening with unfavourable evolution is possible, diagnosis of hypereosinophilic syndrome (HES) should not be delayed until other diagnoses have been excluded, and should be as fast as acute coronary syndrome exclusion.
- If suspicion of HES is high, corticosteroid therapy should be initiated promptly, even before myocardial biopsy or cardiac magnetic resonance imaging (CMRI), because early therapy may prevent significant restrictive heart failure.
KEYWORDS
Hypereosinophilic syndrome, acute coronary syndrome, heart failure, cardiac magnetic resonance.
INTRODUCTION
Hypereosinophilic syndrome (HES) is defined as peripheral blood hypereosinophilia (HE) (blood eosinophils >1.5×109/l) with eosinophil-mediated organ dysfunction or damage and other causes of organ damage excluded. This new wider definition does not require a previous 6-month period of HE, and includes patients with identified aetiologies and those who have not developed signs and symptoms at the time of diagnosis[1].
Cardiac involvement is a major cause of morbidity and mortality, and affects about 50% of patients[2,3]. Clinical manifestations include signs and symptoms of heart failure, intracardiac thrombus, myocardial ischaemia, arrhythmias and, less frequently, pericarditis[2]. The outcome is variable and depends on the progression of endomyocardial fibrosis, with an estimated 5-year mortality of 30%[2]. Clinical presentation of HES simulating a non-ST elevation myocardial infarction (NSTEMI) is rare and so HES is not usually suspected in this scenario.
CASE REPORT
The authors present the case of a 69-year-old female patient admitted to the coronary intensive care unit with a clinical presentation of acute coronary syndrome (ACS), with criteria for NSTEMI: atypical chest pain, ST depression and T wave inversion in V3–V5 on electrocardiogram (EKG), and a serum troponin T level of 3.87 ng/ml (normal range 0.00–0.07 ng/ml). Transthoracic echocardiography revealed left ventricle diastolic dysfunction and coronary angiography showed a 30% distal lesion on the anterior descending artery and a 30% distal lesion on the right coronary artery, which did not confirm the ACS. Moreover, blood tests revealed eosinophilia of 5.30x109/l. The patient was discharged asymptomatic, under antiplatelet therapy and awaiting cardiac magnetic resonance imaging (CMRI) as an outpatient.
Two months later the patient returned to the emergency department with retrosternal pain, fatigue and dyspnoea. The EKG still showed ST depression and T wave inversion in V3–V5 with isolated elevation of the troponin T level (2.12 ng/ml). The eosinophilia had increased to 11.76×109/l. The patient was admitted for further evaluation. A search for secondary causes of the HE showed no evidence of parasitic infections, negative serology for toxoplasma and human immunodeficiency virus (HIV), and negative antinuclear antibodies, cANCA and pANCA.
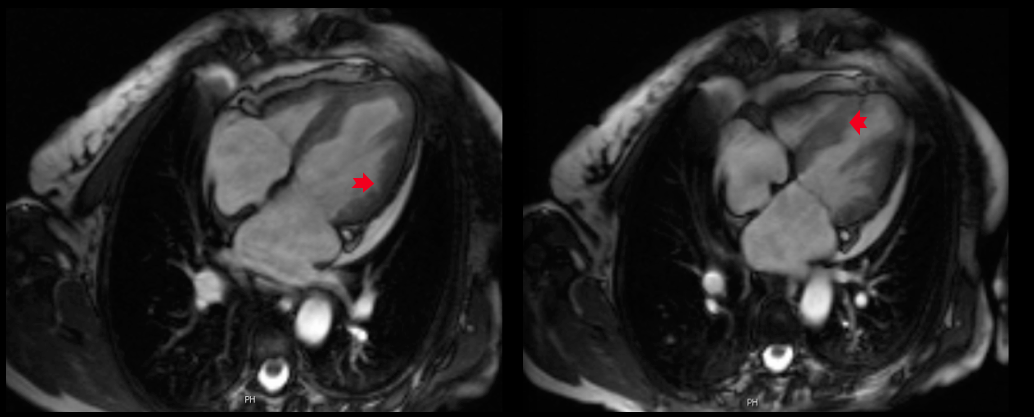
On day 6, the patient’s condition suddenly worsened with symptoms of acute heart failure, presenting a new left bundle branch block on EKG, severely depressed left ventricle function (ejection fraction 29%) and an E/E' ratio of 20, indicating failure of the left ventricle’s diastolic function, without normal valvular function or documented thrombus. There was no initial response to conventional heart failure therapy and prednisolone was initiated (1 mg/kg). Bone marrow biopsy and flow cytometry excluded haematological neoplasia (normal T cell population and no clonal cells). Cytogenetic analysis demonstrated a normal karyotype. An endomyocardial biopsy performed on day 9 of prednisolone therapy, was inconclusive, showing some extravascular lymphocytes. The CMRI revealed an endomyofibrosis pattern (with extensive areas of subendocardial delayed enhancement involving the left ventricle) highly suggestive of eosinophilic myocarditis (Fig. 1).
Figure 1: Cardiac magnetic resonance imaging in a four-chamber view with delayed-enhancement gadolinium images of the left and right heart cavities at the end of diastole (left) and systole (right).
The ejection fraction was 40% with hypokinesia of the anterior wall of the left ventricle, and no thrombus was found. Additional investigation excluded involvement of other organs.
The patient was discharged asymptomatic 3 weeks later, with a normal troponin level and a normal EKG. Outpatient therapy included tapering doses of oral prednisolone and heart failure therapy with beta-blockers, angiotensin converting enzyme inhibitors and a diuretic. One month after admission the patient was asymptomatic, presenting with a normal eosinophilic count and heart function recovery, with an ejection fraction of 46% and just a slightly depressed diastolic function with a E/E' of 16. After 1 year of follow-up, the patient remained asymptomatic, with a normal eosinophilic count and just a slightly depressed diastolic function, and was continuing with conventional heart failure therapy.
DISCUSSION
HES includes all clinical presentations in which serum HE can be documented and there is organ damage as a direct consequence of eosinophilic infiltration, regardless of whether HE is secondary to a reactive process, neoplastic process or another underlying disease. Currently, HES is divided into primary neoplastic HES, secondary HES and idiopathic HES[1]. In the present case, idiopathic HES was assumed because there was no evidence of cause.
When eosinophilia presents with cardiac involvement, some causes of eosinophilic myocarditis must be excluded in the differential diagnosis, such as allergic disorders, hypersensitivity to drugs (some antibiotics, anticonvulsants and antipsychotics), parasitic infections (often protozoal infections such as Trypanossoma cruzi, Toxoplasma gondii and Trichinella spiralis), infectious diseases (HIV or human T cell lymphotropic virus), malignancy (acute or chronic eosinophil leukaemia, T cell lymphoma, and some solid tumours involving the lungs and colon) and systemic vasculitis, especially Churg-Strauss syndrome[4].
Typically, cardiac involvement has three different pathological stages: the necrotic stage, the thrombotic stage and the fibrotic stage. Some patients remain asymptomatic until the latter, when they develop a restrictive or dilated cardiomyopathy[3].
The initial presentation of HES may be unspecific, and in most cases, dyspnoea is the only symptom. Chest pain, cough and palpitations have also been reported. Cardiac involvement most frequently presents as heart failure, intracardiac thrombus, myocardial ischaemia, arrhythmias or pericarditis[2].
There are some reported cases of eosinophilic myocarditis clinically presenting as ACS[5-7]. The clinical manifestations mimicking an ACS may be related to coronary artery spasm, coronary aneurysms, occlusive coronary thrombi or coronary artery dissection[7]. EKG frequently shows T-wave inversion and ST-T wave abnormalities, probably due to endomyocardial fibrosis and inflammation[3]. The serum troponin level increase may indicate the initial phase or the necrotic stage, with normalization after initiation of high dose corticosteroids, which may suggest that troponin T might be a sensitive marker for cardiac damage and cardiac decompensation[3].
Endomyocardial biopsy is still the gold standard for evaluating eosinophil infiltration. However, it can cause important complications, such as ventricular perforation and severe arrhythmias. Furthermore, endomyocardial biopsy has poor sensitivity (about 50%) because of significant sampling error, related to segmental inflammatory infiltration, and variability in the interpretation of biopsy results, which can compromise its effectiveness[5,6]. Despite the need to establish a histological diagnosis, prompt initiation of corticosteroid therapy is mandatory since any delay increases the risk of significant restrictive heart failure[6]. As reported in several cases[3,6,7], CMRI has an emerging important role in the non-invasive diagnosis of cardiac involvement in HES. Delayed-enhancement gadolinium imaging is typically seen in these cases, revealing myocardial inflammation and fibrosis, with high sensitivity in all stages of endomyocardial damage[6].
The management of HES includes conventional therapy for heart failure and immunosuppressive therapy for the underlying eosinophilia. Several cases have shown good outcomes with prednisolone prescribed at a starting dose of 1 mg/kg/day (as used in conventional heart failure therapy), with improvement of symptoms, increased ejection fraction and resolution of eosinophilia[6,7]. In line with other reported cases, our patient had a good response with prednisolone 1 mg/kg/day, with recovery of ejection fraction. After prednisolone was tapered and then stopped, the patient was still asymptomatic at the 1-year follow up, had stable heart function under treatment and had a normal serum eosinophil count. Follow-up should be maintained in order to control the eosinophil level and reassess the need to restart immunosuppressive therapy. There is no evidence for the use of anticoagulation therapy for prophylaxis of thrombotic complications[2,3].
CONCLUSION
This case report describes a rare, potentially fatal disease; however, early initiation of corticosteroid therapy contributed to the favourable outcome. CMRI in this case had an important role in diagnosis since endomyocardial biopsy was inconclusive.